Courses by Software
Courses by Semester
Courses by Domain
Tool-focused Courses
Machine learning
POPULAR COURSES
Success Stories
Week 10 - Simulating Combustion of Natural Gas.
Theory of Combustion: It is a high-temperature exothermic reaction (redox) between a fuel (reductant ) and an oxidant or the oxidizer to release a large amount of chemically bound energy. A flame is a characteristic indicator of the combustion reaction.lt is a sequence of elementary radical reactions. Solid fuels undergo…
Athiyaman R
updated on 23 Sep 2021
Theory of Combustion:
It is a high-temperature exothermic reaction (redox) between a fuel (reductant ) and an oxidant or the oxidizer to release a large amount of chemically bound energy. A flame is a characteristic indicator of the combustion reaction.lt is a sequence of elementary radical reactions. Solid fuels undergo endothermic pyrolysis to generate gaseous fuel whose combustion supplies the heat required to produce more of such gaseous fuel products. The formation of these products is very complex and many different species such as H, 0, OH, CH3, CH4 are formed in the process. Besides Carbon dioxide and water a large no. of pollutants like Nitrous oxide, Carbon mono oxide, and soot particles are formed. A simple example can be seen in the combustion of Hydrogen and Oxygen into water vapor, a reaction generally used to fuel the rocket engines. The reaction releases 250KJ/mol of heat and reduces the enthalpy at constant temperature and pressure.
Types of combustion models:
1. Based on combustion:
Non-Premixed Combustion: In non -premixed combustion fuel and oxidizer enter the reaction zone in separate streams and are not mixed before combustion.Whereas in premixed combustion reactants are mixed with the oxidizer at the molecular level before burning or combustion takes place.
- Examples: Diesel Internal Combustion Engines and pool fires. This is applied only for turbulent flows by using a pressure-based solver.
a) Premixed Combustion: This type of combustion happens when fuel and oxidizer are mixed before burning. They are mixed at the molecular level. Here the flame front propagates into the unburnt reactions as the combustion takes place.
- Examples: Gas Leak combustion explosions, premixed gas-turbine combustors.
Pressure based solver is used and is applicable for turbulent flows and subsonic flows. This type of flames called detonations or deflagrations or explosions, where the combustible mixture is ignited by a heat source behind a shock wave. The Premixed combustion model cannot be used along with the NOx pollutant model and soot model turned on, whereas the partially premixed model can be used along with the pollutant model turned on. Only inert particles can be used for simulation by the premixed combustion model, not the discrete phase particles.
b) Partially premixed combustion:
Here fuel and oxidizer are primarily mixed before combustion. They are the premixed flames with non-uniform fuel-oxidizer mixtures. It is a model combustion process intended to be used in Internal combustion engines of motorized vehicles and other automobiles. Its high specific power, high fuel efficiency, and low exhaust pollution made it a promising technology. These premixed flames involve premixed jets discharging into the atmosphere, lean premixed combustors with cooling air jets, or diffusion pilot flames. In order to compute the concentrations of individual species, a partially premixed combustion model is used. This is applicable for turbulent flows by the use of a pressure-based solver.
2. Based on Phases:
a) Fluid phase: Combustion happens when fuel is in the fluid phase.
b) Volumetric phase/gas phase: It is for gas-phase reactions. The reaction rates are defined on a volumetric basis.
c) Wall surface: For gas-phase reactions, the reaction rate is defined on a volumetric basis. Here combustion happens on a wall or boundary. For surface reactions, the rate of adsorption and desorption is governed by both chemical kinetics and diffusion process. When the volumetric is enabled, wall surface reactions create sources and sinks of species in the gas phase enabling the calc of wall surface reaction kinetics. They are also applied to the reacting surface.
d) Particle surface: Combustion happens over surfaces involving multi-phase particle surface reaction mechanism. This is applied by using the volumetric turned on.
MODELLING AND MESHING
Geometry:
Only 1/4th part of the model is used for analysis. A body split tool is used for splitting bodies. The 2D front face is used for analysis by a copy-paste tool.
The process of 3d Model to 2D Model


2D geometry for Analysis:

Meshing:
The next step after generating a 2D model is to mesh a model for further simulation.



Named Selections:
After generating optimum fine meshing, the next step is to give name selection to the boundaries for specifying physical properties at particular boundaries. The Name Selections in the model are as shown in the figure below.

SETUP IN FLUENT:
- Solver- Pressure based
- Steady-state Simulation
- 2D-Planar Axisymmetric

- Viscous Turbulence model- K epsilon -> Standard -> Standard wall functions

- Species Model Turned ON

Species:
The Species Model dialog box allows you to set parameters related to the calculation of species transport and combustion.
Species Transport - enables the calculation of multi-species transport (either non-reacting or reacting, depending on the selection for Reactions).
Reactions - contains options related to the modeling of reacting flow.
Volumetric - enables the calculation of reacting flow using the finite-rate formulation.
Inlet Diffusion - includes the diffusion flux of species at the inlet.
Diffusion Energy Source - includes the effect of enthalpy transport due to species diffusion in the energy equation.
Mixture material - Methane-air 2 step is used as it allows the addition of water in fuel or air
NOX and Soot model is turned ON:
1) NOx Model:

Mainly consists of Nitric oxide (NO), Nitrogen peroxide (NO2), and Nitrous oxide (N20) to a smaller extent Thermal, Prompt, Fuel, and N20 intermediate mechanisms are available.
Thermal NOx - It is formed when nitrogen and oxygen in the combustion air combine with one another at the high temperatures in a flame.
Prompt NOx - Forms from the rapid reaction of atmospheric nitrogen with hydrocarbon radicals.
Fuel NOx - It is formed by the reaction of nitrogen bound in the fuel with oxygen in the combustion air. It is rarely a problem with gaseous fuels.
In our case, we will activate Thermal and prompt NOx, as there is no nitrogen in input fuel as is Methane CH4.
2) Soot Model
A One-Step Soot Model is used.

Boundary conditions:
- For Part-I, as there is no addition of Water h2O, the Air inlet will have a velocity of 0.5 m/s with a thermal temperature 300 k with species of 0.23 mass fraction of O2. For Fuel inlet, velocity is 80 m/s with thermal temperature 300 k and species of 1 mass fraction of ch4.
1. Air-inlet: Velocity = 0.5 m/s, T = 300 K, Mass fraction of O2 = 0.23
2. Fuel-inlet: Velocity = 80 m/s, T = 300 K, Mass fraction of CH4 = 1.
3. Outlet: Pressure Outlet, Guage pressure = 0.
4. Walls: Wall (No-slip wall)
- For Part-II, there is an addition of water in terms of 5, 15, 25, and 30% in fuel. so all the above conditions remains same with only change in fuel inlet species with 0.05 , 0.15 , 0.25 , 0.3 mass fraction of h2O with ch4 of mass fraction 0.95 , 0.85 , 0.75 , 0.7 respectively. Air inlet species remains the same with a 0.23 mass fraction of O2.
1. Air-inlet: Velocity = 0.5 m/s, T = 300 K, Mass fraction of O2 = 0.23
2. Fuel-inlet: Velocity = 80 m/s, T = 300 K,
- Mass fraction of CH4 = 0.95, 0.85, 0.75, 0.7 for 5, 15, 25, and 30% of Water in Fuel respectively.
- Mass fraction of H2O = 0.05, 0.15, 0.25, 0.3 for 5, 15, 25, and 30% of Water in Fuel respectively.
3. Outlet: Pressure Outlet, Guage pressure = 0.
RESULTS :
Part-I : CH4 = 1, O2 = 0.23
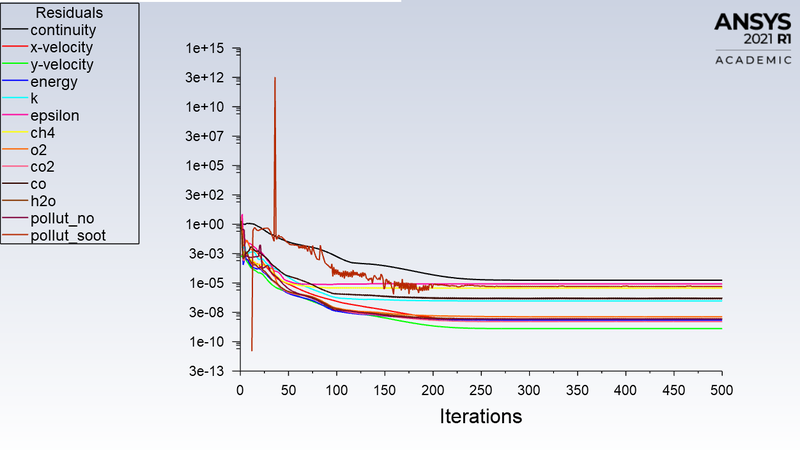
Residuals:

Temperature Contour :

Mass fraction of CH4 along with chart at varying distances:


Mass fraction of CO2 along with chart at varying distances:


Mass fraction of O2 along with chart at varying distances:


Mass fraction of H2O along with chart at varying distances:


Mass fraction of N2 along with chart at varying distances:


Mass fraction of NOx along with chart at varying distances:


Mass fraction of Soot along with chart at varying distances:


Part-II :
Case 1: CH4=0.95, H2O = 0.05, O2 = 0.23 (5% Water in Fuel).
Residuals:
Temperature Contour

Mass fraction of CH4 along with chart at varying distances:


Mass fraction of CO2 along with chart at varying distances:


Mass fraction of O2 along with chart at varying distances:


Mass fraction of H2O along with chart at varying distances:


Mass fraction of N2 along with chart at varying distances:


Mass fraction of NOx along with chart at varying distances:


Mass fraction of Soot along with chart at varying distances:


Case 2: CH4=0.85, H2O = 0.15, O2 = 0.23 (15% Water in Fuel)
Residuals:

Temperature Contour

Mass fraction of CH4 along with chart at varying distances:


Mass fraction of CO2 along with chart at varying distances:


Mass fraction of O2 along with chart at varying distances:


Mass fraction of H2O along with chart at varying distances:


Mass fraction of N2 along with chart at varying distances:


Mass fraction of NOx along with chart at varying distances:


Mass fraction of Soot along with chart at varying distances:

Case 3: CH4=0.75, H2O = 0.25, O2 = 0.23 (25% Water in Fuel).
Residuals:

Temperature Contour
Mass fraction of CH4 along with chart at varying distances:


Mass fraction of CO2 along with chart at varying distances:


Mass fraction of O2 along with chart at varying distances:


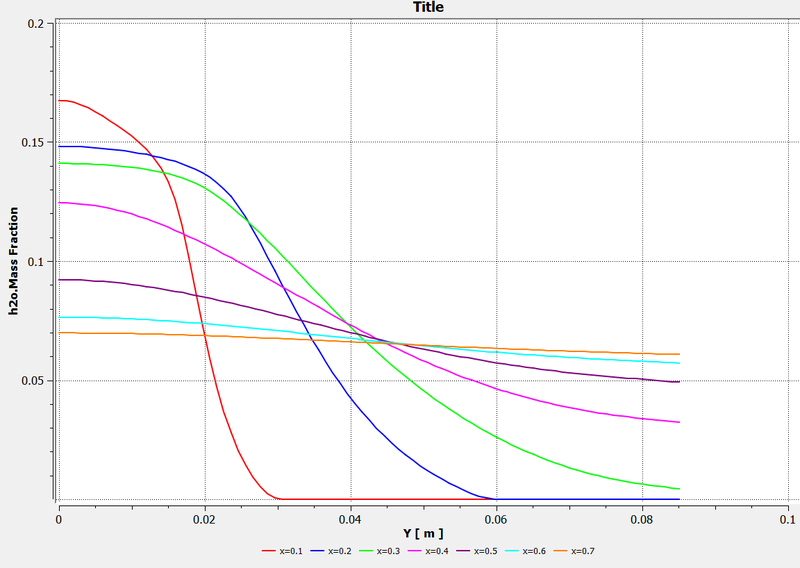
Mass fraction of H2O along with chart at varying distances:

Mass fraction of N2 along with chart at varying distances:


Mass fraction of NOx along with chart at varying distances:


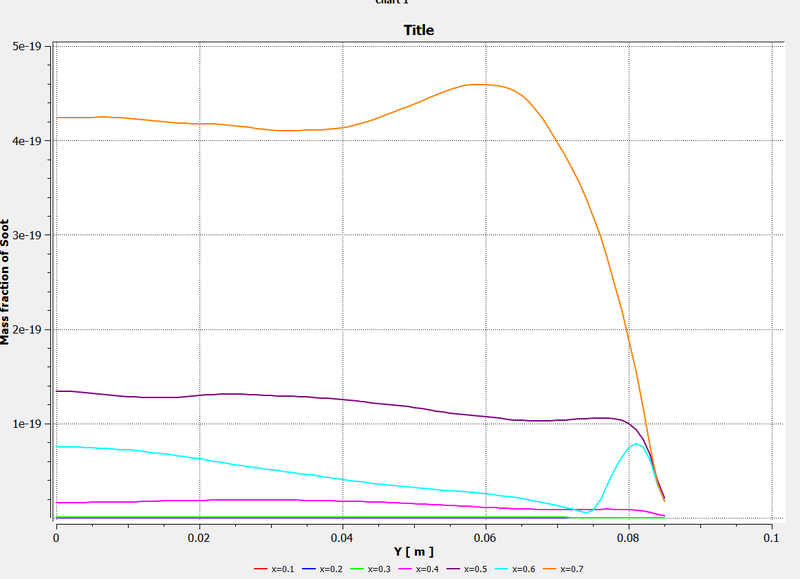
Mass fraction of Soot along with chart at varying distances:


Case 4: CH4=0.7, H2O = 0.3, O2 = 0.23 (30% Water in Fuel).
Residuals:

Temperature Contour

Mass fraction of CH4 along with chart at varying distances:


Mass fraction of CO2 along with chart at varying distances:


Mass fraction of O2 along with chart at varying distances


Mass fraction of H2O along with chart at varying distances:


Mass fraction of N2 along with chart at varying distances:


Mass fraction of NOx along with chart at varying distances:


Mass fraction of Soot along with chart at varying distances:


Conclusion:
After running the simulation for both the parts, there are few conclusions that can be drawn out. The parts consist of different setup with the first being without the addition of H2O in Fuel and the second being with the different proportion of water in fuel from 5% to 30%.
1) As can be observed from the first part of a simulation, the pollutant NOx and soot are getting formed at the outlet of the combustor as can be seen in the graphs and table above. Such formation has harmful effects on the environment and humans. The stringent government norms also demand the least formation of Nox and soot and to satisfy those requirements, it needs to check the effect of adding the water in the fuel.
2) From graphical representations, we can see that in the second part, the value of the mass fraction of NOx decreases with an increase in addition to water (H2O) in Fuel, which is great according to government norms with a reduction of NOx formation.
3) We can also observe that the mass fraction of soot reduces with an increase in the addition of water. Thus we can conclude that Combustion simulation is performed successfully.
Leave a comment
Thanks for choosing to leave a comment. Please keep in mind that all the comments are moderated as per our comment policy, and your email will not be published for privacy reasons. Please leave a personal & meaningful conversation.
Other comments...
Be the first to add a comment
Read more Projects by Athiyaman R (16)
Week 10 - Simulating Combustion of Natural Gas.
Theory of Combustion: It is a high-temperature exothermic reaction (redox) between a fuel (reductant ) and an oxidant or the oxidizer to release a large amount of chemically bound energy. A flame is a characteristic indicator of the combustion reaction.lt is a sequence of elementary radical reactions. Solid fuels undergo…
23 Sep 2021 08:31 AM IST
Week 9 - Parametric study on Gate valve.
Introduction The gate valve is the fluid valve that opens by lifting the barrier gate from the path of the fluid flowing thriugh a pipeline. Gate valves require generally very less space and are and can be fitted along the pipe axis and they hardly restict any fluid flow when they are fully opened. The schematic of the…
06 Sep 2021 05:19 AM IST
Week 8 - Simulating Cyclone separator with Discrete Phase Modelling
Empirical models for Cyclone Separator Efficiency: Iozia and Leith Model: Iozia and Leith logistic Model is a modified version of Barth (1956) model which is developed based on force balance. The model assumes that a particle carried by the vortex endures the influence of two forces: a centrifugal force Z, and flow resistance,…
26 Aug 2021 01:44 PM IST
Week 6 - CHT Analysis on a Graphics card
Conjugate Heat Transfer (CHT) Conjugate heat transfer is defined as the heat transfer between two domains by exchange of thermal energy. For a system the thermal energy available is defined by its temperature and the movement of thermal energy is defined by its heat flux through the outer walls. Heat transfer in…
02 Jul 2021 05:34 PM IST
Related Courses


Skill-Lync offers industry relevant advanced engineering courses for engineering students by partnering with industry experts.
Our Company
4th Floor, BLOCK-B, Velachery - Tambaram Main Rd, Ram Nagar South, Madipakkam, Chennai, Tamil Nadu 600042.
Top Individual Courses
Top PG Programs
Skill-Lync Plus
Trending Blogs
© 2025 Skill-Lync Inc. All Rights Reserved.